Pyrolysis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
smoke is just the last unburned and condensing residual wood vapors, released before the match cools sufficiently to stop outgassing them. [https://www.biochar-journal.org/itjo/media/doc/1437139451142.pdf] | smoke is just the last unburned and condensing residual wood vapors, released before the match cools sufficiently to stop outgassing them. [https://www.biochar-journal.org/itjo/media/doc/1437139451142.pdf] | ||
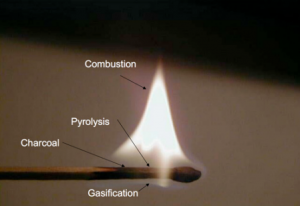
[[File:Match pyrolysis.png|thumb|Physical/chemical diagram of a match on fire.]] | |||
https://permanent.fdlp.gov/gpo129286/fpl_gtr270.pdf | https://permanent.fdlp.gov/gpo129286/fpl_gtr270.pdf | ||
Revision as of 19:04, 29 April 2022
Although it seems counterintuitive, wood actually does not burn. Instead it is the gas emitted by heating the wood that burns. Only when the wood is finally charred under the flame of the woodgas, can oxygen penetrate the then porous structure of the newly charred wood and glow the carbon to ash. Striking a match on the rough surface of the matchbox ignites a flaming chemical reaction of the sulfurous tip that generates enough heat to make the wood emit highly combustible gases. The flame ignites the gases so released from the wood and the process continues under the heat from the burning pyrolysis gases, causing further outgassing and burning of gas. But underneath the flame of the woodgas the wood itself does not burn but carbonizes, because the gas flame consumes all the oxygen, creating a pyrolysis zone where the flame protects the match from oxidation. As we know, the match burns with a clean flame until someone blows out the flame after which it will smoke. The smoke is just the last unburned and condensing residual wood vapors, released before the match cools sufficiently to stop outgassing them. [1]